-
Configurationally Stable Doubly Bridged Biphenyl Azocines through Copper-Catalyzed Double Carbene Insertions into the Corresponding Azepines
S. Harthong, E. Brun, S. Grass, C. Besnard, T. Bürgi and J. Lacour
Synthesis, 48 (19) (2016), p3254-3262


DOI:10.1055/s-0035-1562467 | unige:86678 | Abstract | Article HTML | Article PDF | Supporting Info
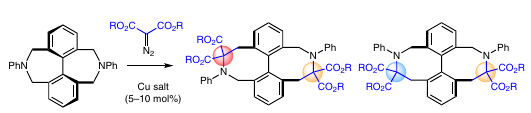
Doubly bridged biphenyl azocines can be prepared in a single step through copper-catalyzed reactions of a doubly bridged biphenyl azepine and diazodiester reagents. Double [1,2]-Stevens rearrangements occur at 100 °C to afford doubly tethered eight-membered rings (49 to 61%) as trans and cis regioisomers (1:1 ratio). These products present an axial chirality. ECD and VCD analyses of the separated enantiomers (CSP-HPLC) were used to assign the absolute configuration. High configurational stability is observed for both regioisomers as racemization does not occur after 1 week of heating at 208 °C in dodecane (ΔG ‡ > 41 kcal·mol–1). Interestingly, reactions performed at 40 °C retain a certain level of enantiospecificity (82–86%), avoiding, for the most part, thermal racemization of the starting material.